
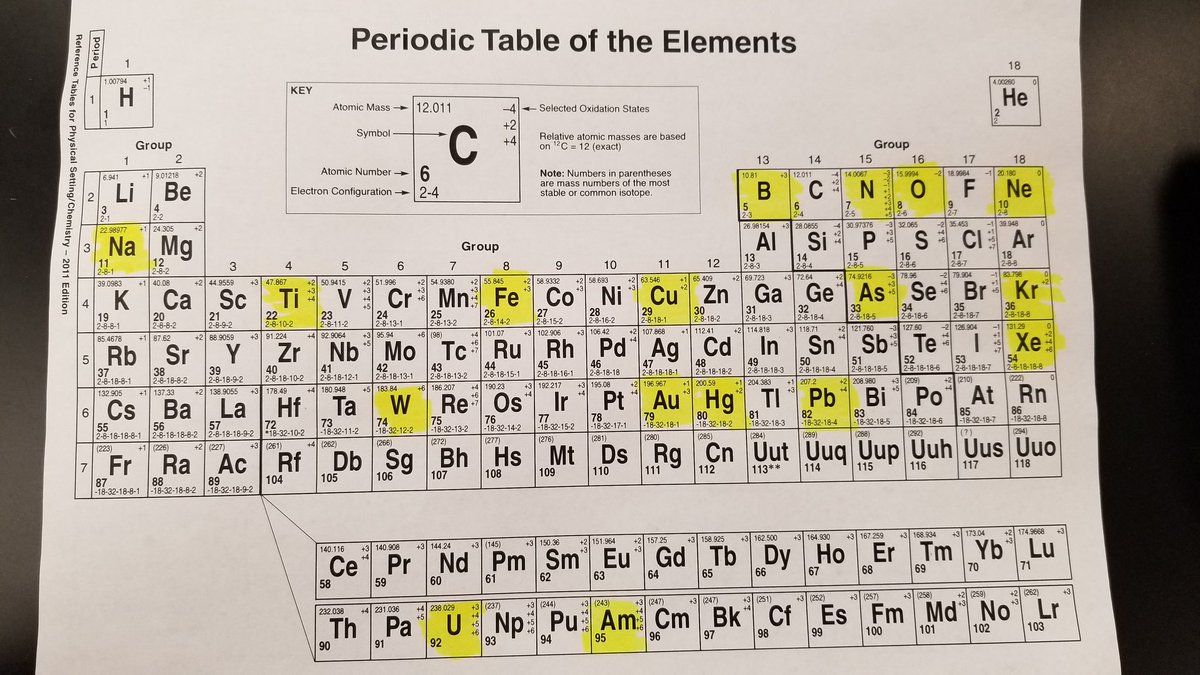
The top of each element’s informational table displays a Bohr model of that element, while the bottom includes a link to a Google search of the same. Every element comes with its own fun fact, such as yttrium’s presence in lunar rock and how no one really knows what roentgenium looks like. Users can click on an element to learn its year of discovery, melting and boiling temperatures, density, and atomic mass. (The cool thing about legacy scientific reference tables is that they shouldn’t really vary from place to place.) Beneath the surface, however, Google’s searchable classroom poster contains a handful of useful features. The table is simple and unintimidating at first glance. This topic is discussed across several sections of this book.(Photo: Vedrana Filipovic/Unsplash) Remember how teachers always told us we wouldn’t have a calculator in our back pocket, and then smartphones ruined that particular excuse for learning math? Google is doing the same with the elements through their new interactive periodic table, which allows users to browse and learn about the stuff that makes up other stuff.
In these situations, electrons get Z = −1 and neutrons get Z = 0. The atomic number can also be applied to things like electrons and isolated neutrons in nuclear reactions. It is always a whole number greater than zero when used to identify an element. Each element can be identified from this number alone. It is represented by the symbol Z in equations because Z is the first letter in the word atomic number (definitely not true). The atomic number is the number of protons inside the nucleus of an atom. Two numbers are usually added to each cell. I can fill my periodic table with placeholder names and symbols and then replace them with real names and symbols as reality reveals itself to science. As a pleasant side effect, the systematic naming convention also makes it possible to name elements that haven't been synthesized yet (and even those that may never be synthesized). If laboratory A says it's found a way to synthesize element X and laboratories B and C can't synthesize element X using the same process, no one gets to name an element that day. This gives the scientific community time to verify claims of discovery. Naming elements is a much slower process now as a result. The International Union of Pure and Applied Chemistry (IUPAC), which establishes rules for things like naming chemical elements, invented systematic names to impose a kind of armistice on warring chemists. Naming rights go to the lab with priority in a discovery. Put the two together and you get Transfermium Wars - a bunch of chemists arguing about whose laboratory (and by proxy, whose superpower nation) was the best. At this time, nuclear chemists in the United States and the Soviet Union were synthesizing elements heavier than fermium (thus the adjective "transfermium") and the governments of the United States and the Soviet Union were embroiled in the Cold War (thus the noun "wars"). This last convention arose during the Transfermium Wars of the late 20th century, which, despite sounding like a science fiction battle for supremacy of the galaxy, was actually nothing more than an academic argument. How this scenario plays out is open to some speculation.
Some have proposed naming it feynmanium (Fy) after the American physicist Richard Feynman who predicted it would be the heaviest element possible. If and when it is discovered it will be called this for a while.
#PERIODIC TABLE CHEMISTRY REFEREWNCE YTABLES HOW TO#
Are you still here? Well, I guess I should tell you something about the periodic table - like how to read it.


 0 kommentar(er)
0 kommentar(er)
